Molarity is often used to express concentrations of plant hormones especially in tissue culture.
It is a good way to compare hormones because it is an expression of active molecules in the solution regardless of the weight of the compound.
A one molar solution (1 M) is the molecular weight of the compound in grams dissolved in a solvent to measure one liter.
Researchers commonly use molarity to express hormone concentrations.
Growers need to be able to convert molar to ppm as well as ppm to molar.
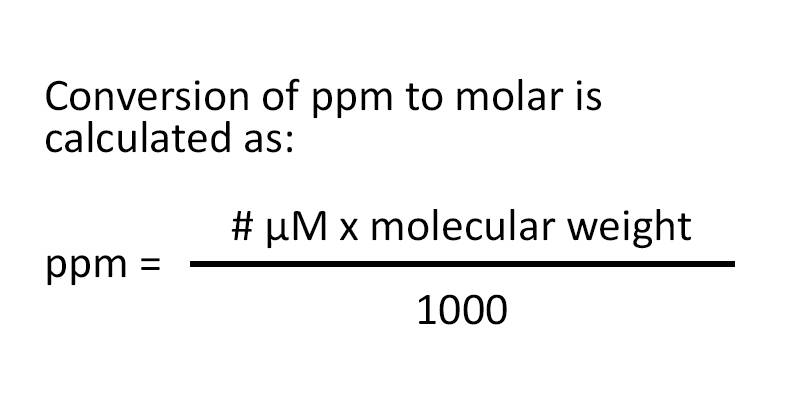
Conversion of ppm to molar uses the following formula:
ppm = micromolar concentration x molecular weight divided by 1000.

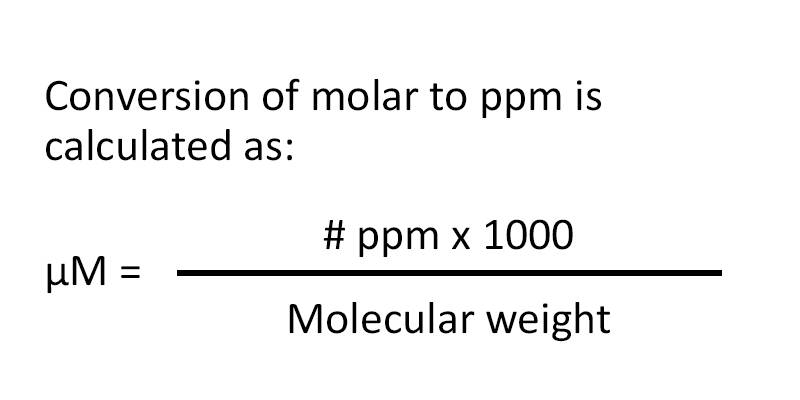
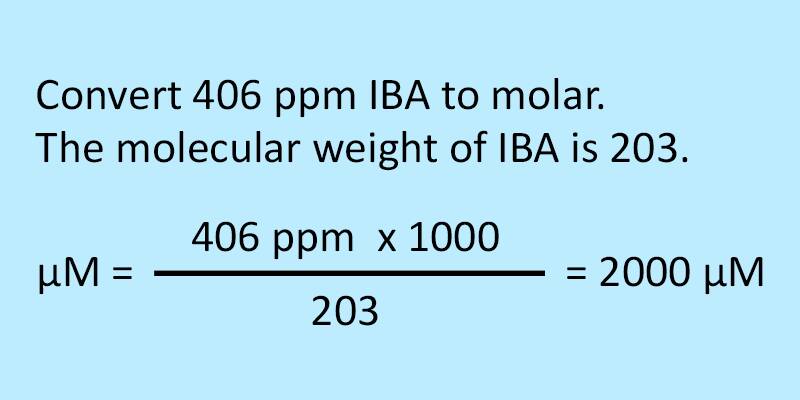
Conversion of molar to ppm uses the following formula:
Micromolar = (ppm x 1000) divided by molecular weight.