One of the first hormone responses in plants was the observation that light affected the direction of growth of plant coleoptiles in germinating seeds.
In the early 1900's, Fritz Went and a number of other researchers showed that these effects could be induced by plant extracts, that were subsequently shown to contain the plant hormone indoleacetic acid (IAA).
Auxins are commonly used as an aid to rooting cuttings.

Auxins promote adventitious root formation as shown with these yew (Taxus) cuttings.
Auxin is the most important hormone involved in rooting.
Naturally Occurring
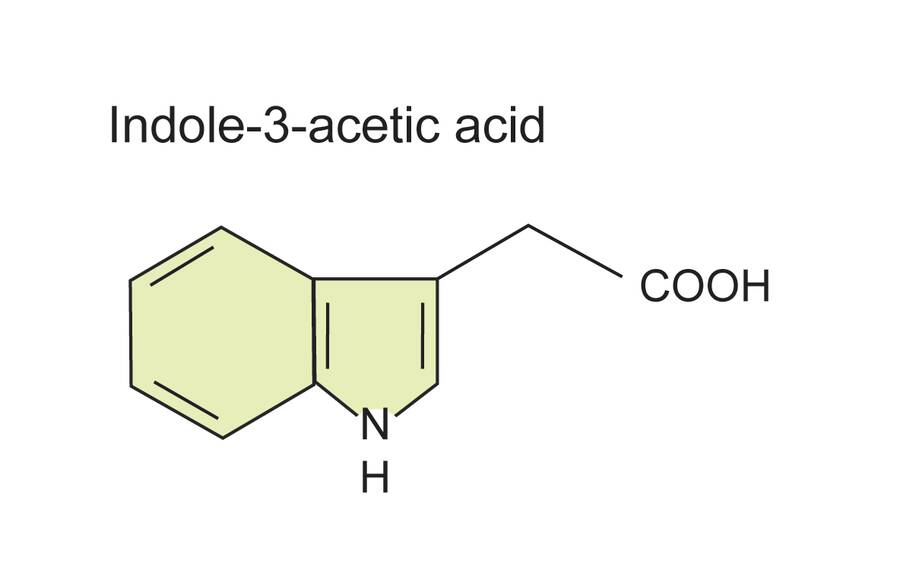
Indole-3-acetic acid (IAA)
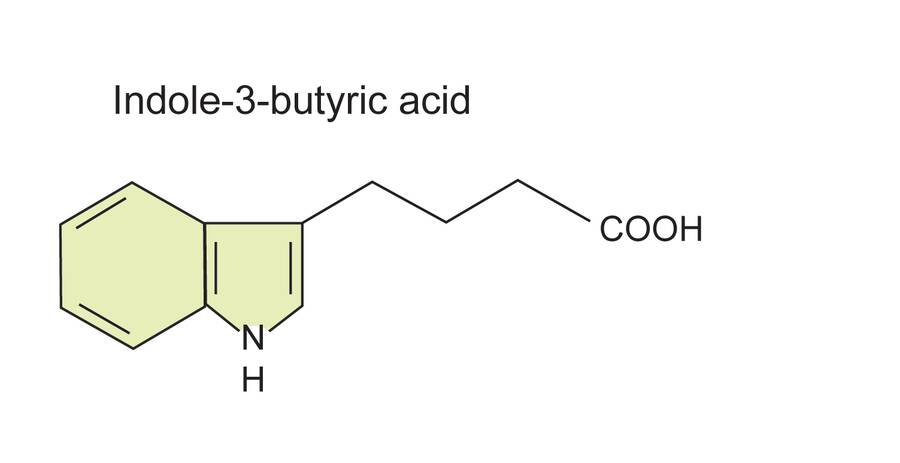
Indole-3-butyric acid (IBA)
Synthetic
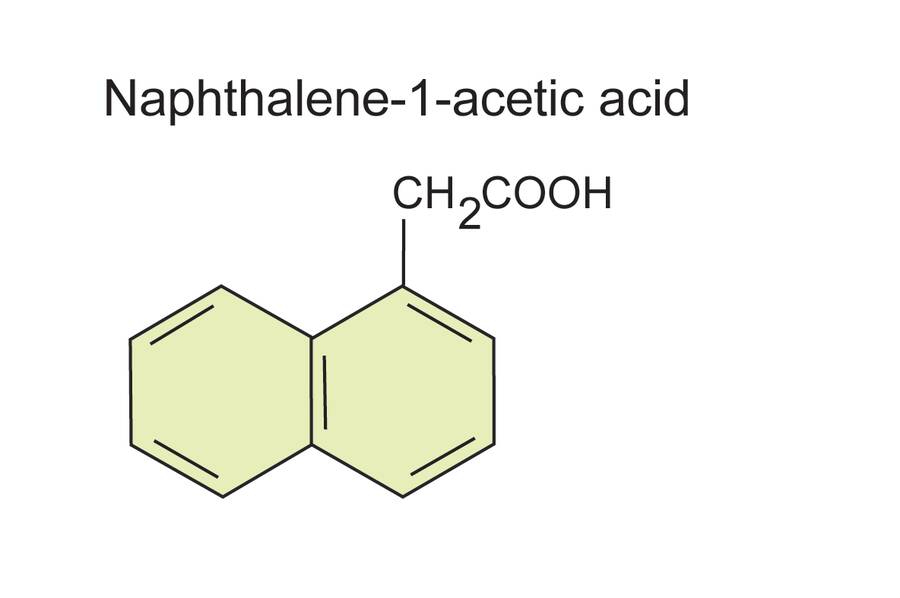
α-Naphthalene acetic acid (NAA)
2,4-diclorophenoxyacetic acid (2,4-D)
IAA is the most abundant naturally occurring auxin in plants. It is synthesized from the amino acid L-tryptophan in leaf primordia, young leaves and developing seeds.
Although naturally occurring, IAA is not commonly used in propagation because it breaks down quickly in the plant.
Synthetic auxins like IBA and NAA are more commonly used and are more effective.
Indole-3-butyric acid (IBA) is naturally occurring, but at very low abundance.
It works by being converted to IAA by the plant.
It is commonly found in commercial rooting compounds.
α-Naphthalene acetic acid (NAA) is a purely synthetic auxin.
It is chemically similar to IAA in structure but is a more effective auxin in promoting rooting.
It is commonly found in commercial rooting compounds and is often combined with IBA.
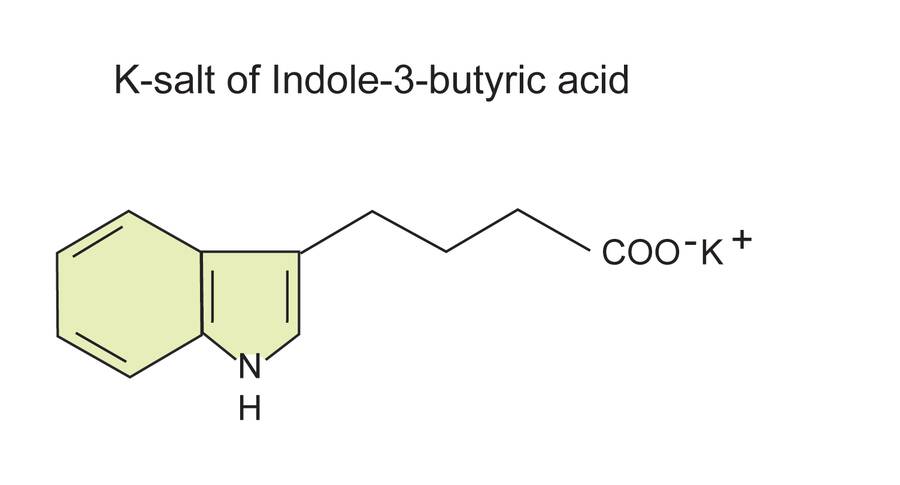
The potassium salt form of IBA and NAA have the advantage of being water soluble and not needing a solvent.
In some cases IBA or NAA treated cuttings can be damaged by the solvent used to dissolve them.
In these cases, the water soluble K-IBA and K-NAA can be a very useful alternative.
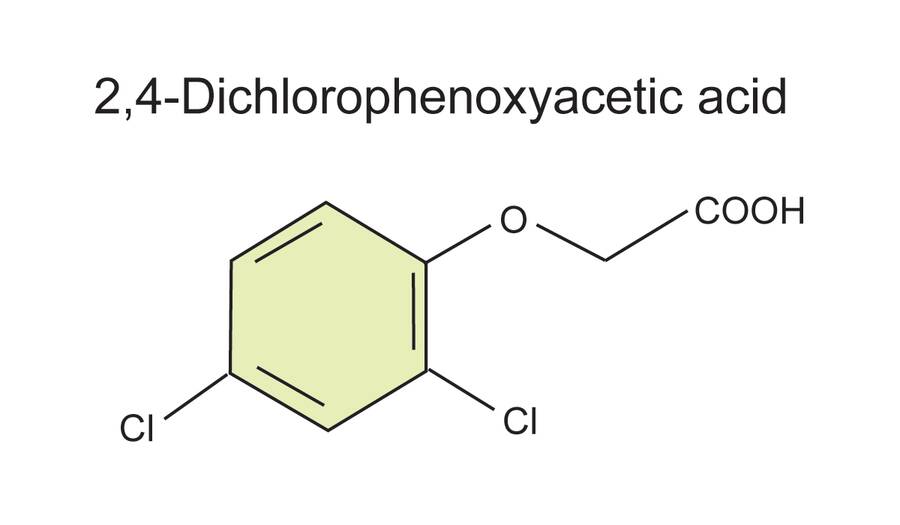
The herbicide 2,4-dicloro-phenoxyacetic acid (2,4-D) is also considered a synthetic auxin, but it is seldom used commercially to promote rooting.
It is commonly used to induce somatic embryogenesis in tissue culture.
IAA is not used commercially as often as synthetic auxins.
This is because it is not as stable.
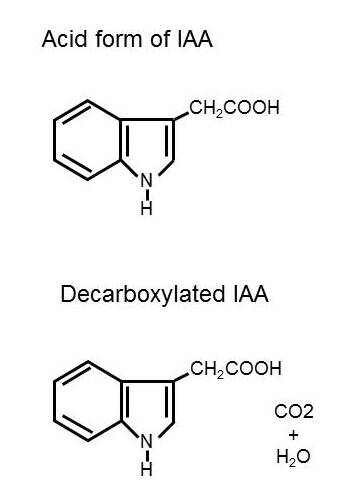
IAA degrades in the light and is susceptible to destruction in the plant by IAA-oxidase.
IAA-oxidase removes the carboxyl group (COOH) making it ineffective as an auxin.
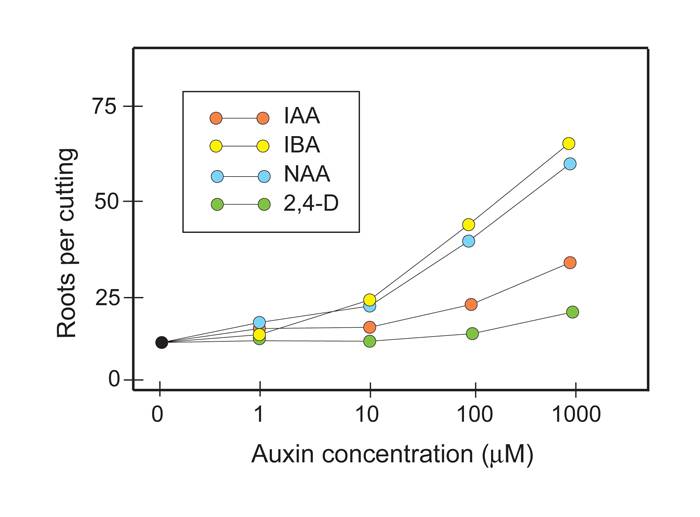
Relative rooting response of different auxins.
Conjugation of IAA naturally protects it from destruction by decarboxylation.
Conjugation adds a sugar or an amino acid to the carboxyl end of the molecule.
The conjugated form can be metabolized back to active IAA.
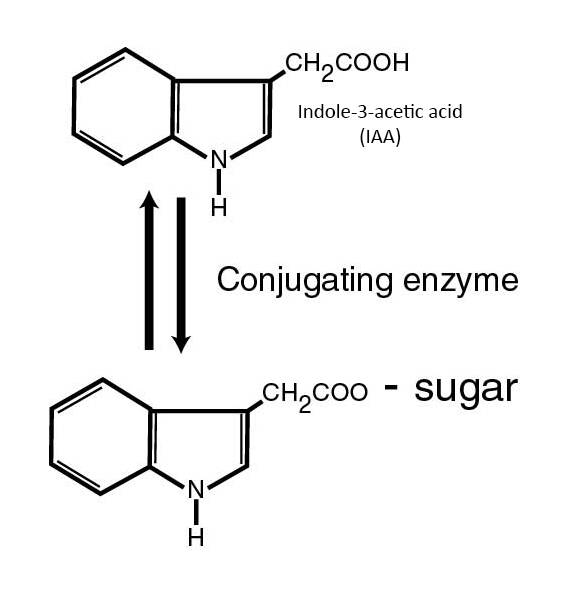
There are conjugated forms of auxin used for cutting propagation.
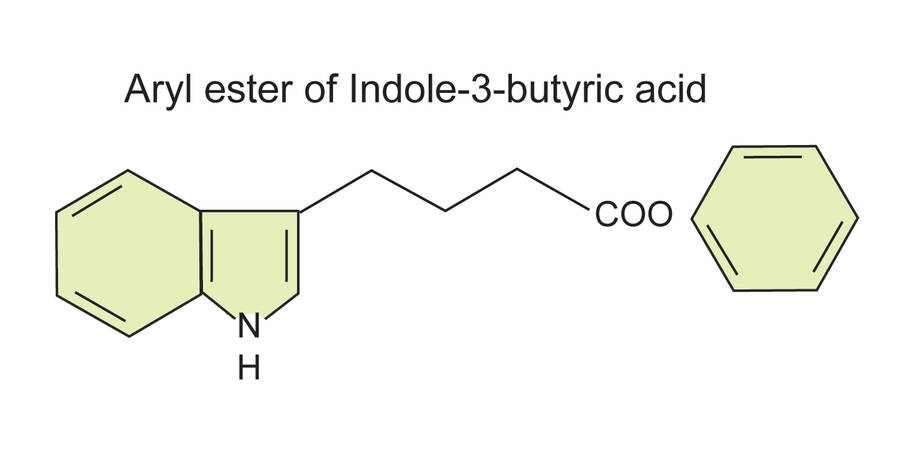
These are also aryl ester and aryl amide forms of IAA and IBA called phenyl-IAA (P-IAA), phenyl-IBA (P-IBA), phenyl thioester IBA (P-ITB) and phenyl amide IBA (NP-IBA).
These have been shown to be effective alternatives to IAA and NAA and show less toxicity in animal studies. P-ITB has label clearance from EPA, but is still not commonly available.
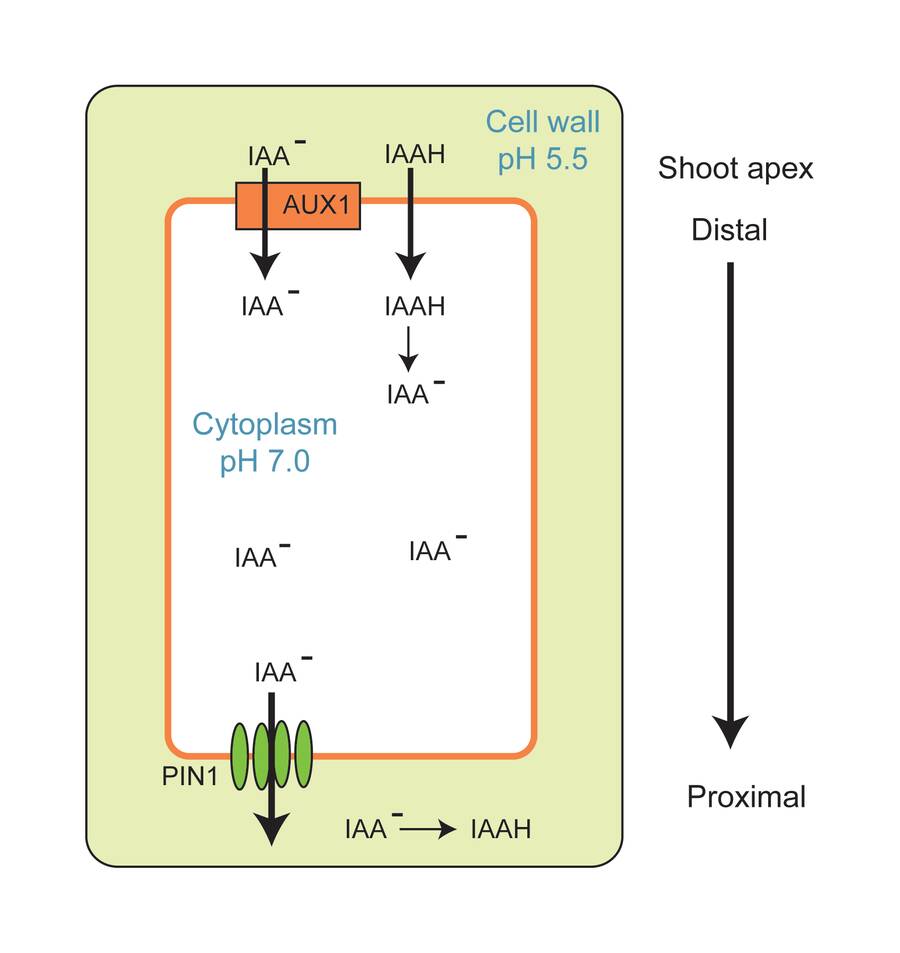
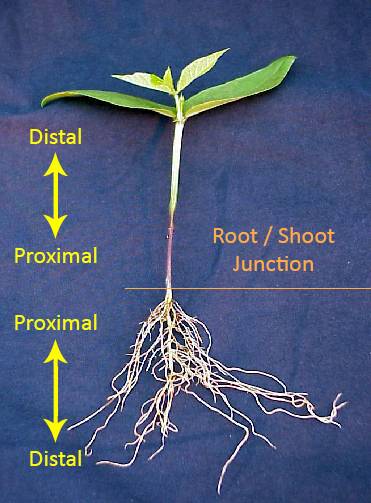
Auxin is produced in the apical meristems.
Auxin moves from cell to cell in a polar gradient (i.e., tip to base).
It moves from distal to proximal.
This is why cuttings root at the base (proximal end) of the stem.
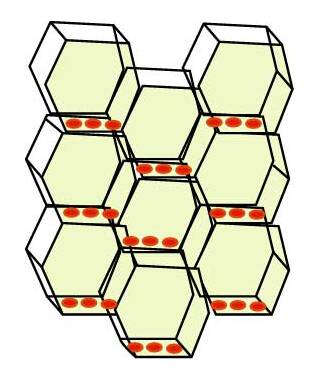
Auxin transport proteins are located at the base of parenchyma cells.
Transport is not sensitive to gravity and always moves in a polar direction.
Polar auxin transport
Transporter proteins (PIN1) are only located at the proximal end of the cell.
Therefore, auxin can only move in one polar direction.
Mutations in the PIN protein result in embryos with poorly formed meristems showing how important polar transport and auxin gradients are for plant growth and development.